
Earth & Space Science
Ready-to-go kits and our most popular science products.
Plus, free hands-on activities, guides, and printable posters.
Ward's Preserved Specimens
The Widest Selection of Preparations, Backed by Expert Support

Physics
From classic and cutting-edge apparatus and demos, to time-saving activities, durable equipment, and essential supplies for your lab - we have what you need to turn physics lessons into physics connections.
Explore, Discover and Inspire
Explore our popular hands-on resources that support your science lesson plans. Discover how Boreal Science makes it easy for you to reinforce skills and help students make sense of the world around them. Browse the subjects below to find featured products that inspire students to ask questions, collect information, organize and test ideas, solve problems, and apply what they learn.
You can also browse popular resources or shop now for specific products.
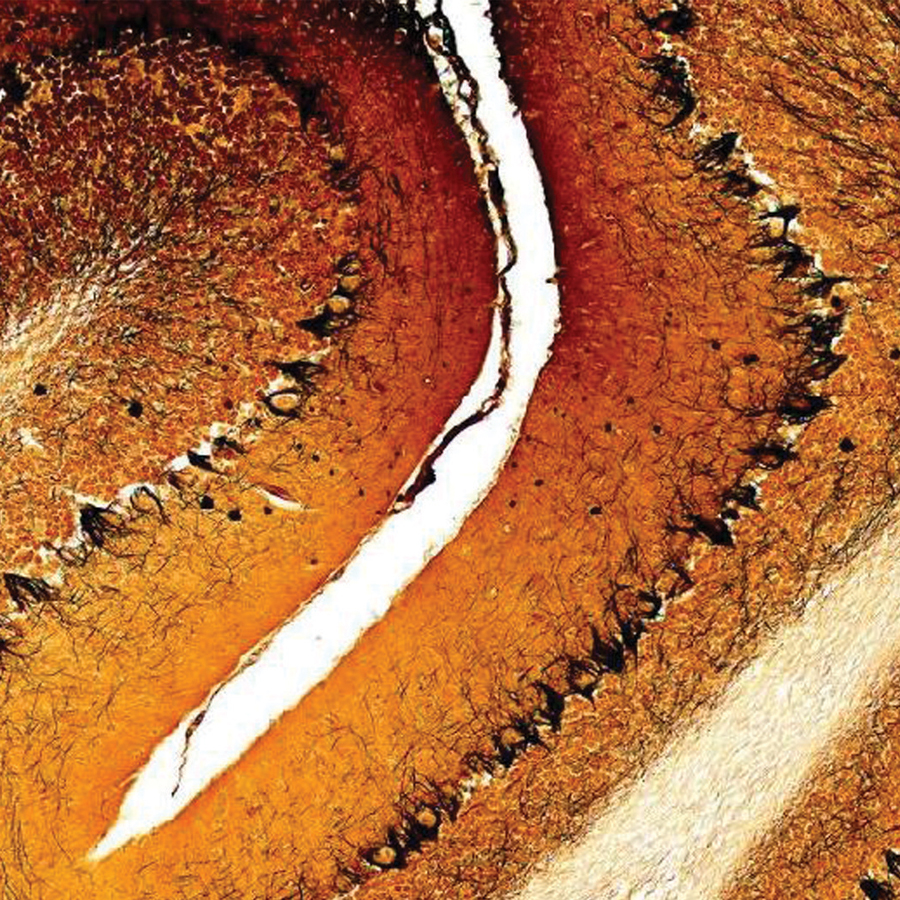
Biology & Life Sciences
Browse kits, specimens, guides, and videos that inspire students to learn biological concepts.
Browse Our Most Popular
Shop Now

Earth & Space Science
Explore activities, equipment, and models that support lesson plans about Earth and neighboring planets.
Shop Now

Chemistry
Check out supplies, chemicals, activities, and models to stock your lab and educate students.
Shop Now

Physics
Discover apparatus, demos, robotics, supplies, and equipment that help demonstrate the properties and laws governing space, time, energy, and matter.
Shop Now
More to Explore!
Stock your lab for success with equipment and supplies for every application and budget.
Discover high quality, affordable microscopes for beginner to advanced study, with options for every application.






